Intubation Pharmacology
Listen to Lecture
The intubation drugs can be divided into three classes:
- Induction agents – causes unconsciousness so that the patient is unaware of the procedure and the physiological response to the stimulus of laryngoscopy is reduced.
- Muscle relaxants – relaxes the muscle tone and abolishes the protective airway reflexes allowing laryngoscopy and causes the vocal cords to relax in the open position facilitating passage of the endotracheal tube.
- Rescue medications – drugs to deal with common complications caused by the process of intubation or by the administration of the induction agents e.g. bradycardia and hypotension.
As well as discussing each of these in detail, this chapter will also provide advice on post intubation drug administration.
Induction Agents
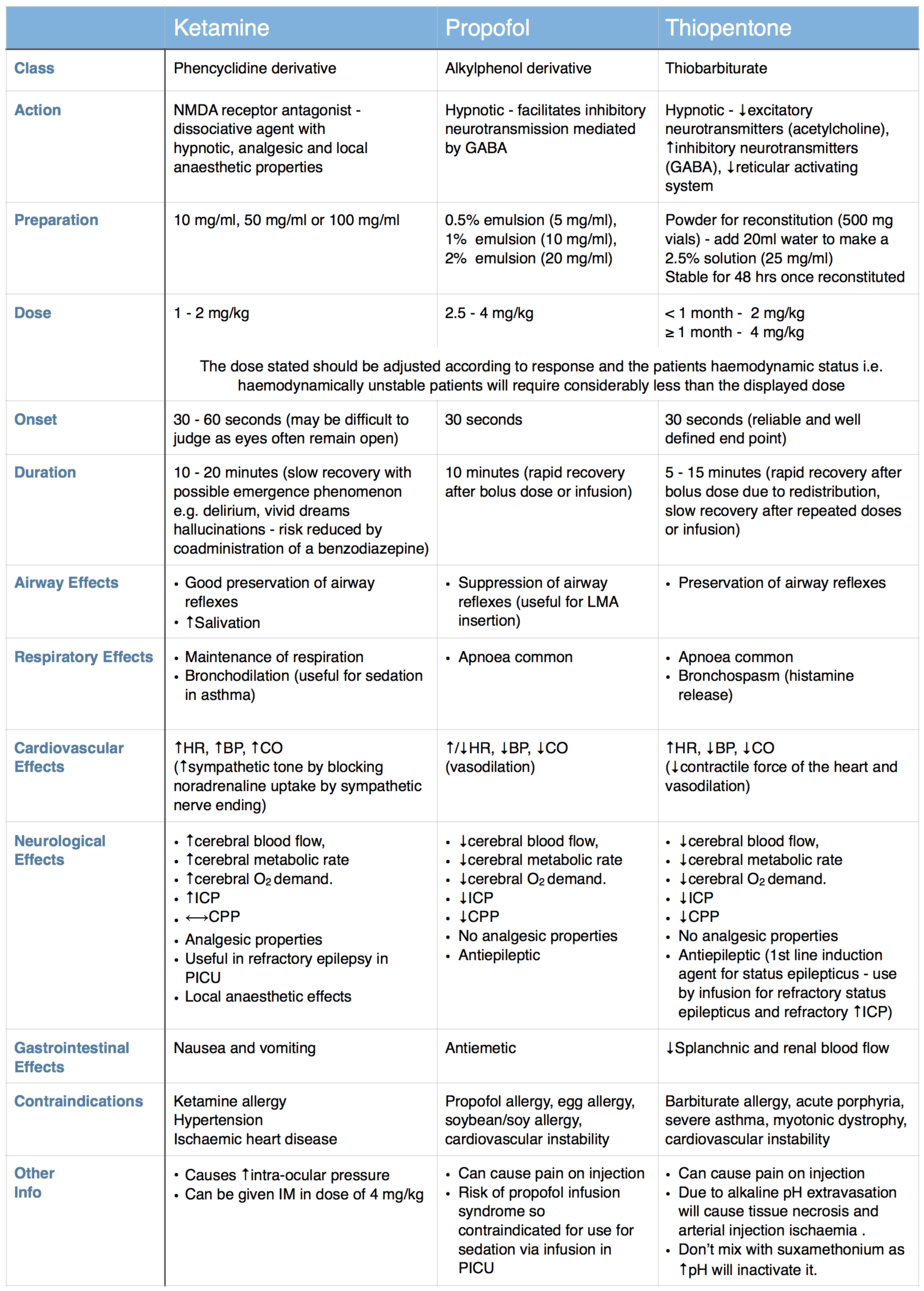
A wide variety of induction agents are used routinely in children and it is important to know their mechanism of action, individual advantages and common side effects, so that the most appropriate agent is used in each case. The properties of three of the most commonly used induction agents are compared in the table below.
Other Induction Agents
Etomidate is a cardiac stable induction agent, however its use is associated with increased mortality in critically ill patients (it causes suppression of adrenocortical function), and therefore it’s use cannot be recommended.
Fentanyl a synthetic opioid, while not truly an induction agent, is often given in high doses of 3 – 5 mcg/kg for this purpose in neonatal units or during cardiac anaesthesia (normal analgesic dose = 1 mcg/kg). It is normally well tolerated from a cardiovascular point of view and the main side effect is chest wall rigidity. This risk can be minimised by giving the fentanyl slowly over approximately 2 minutes (remember during initial administration you are only filling the dead space of the line and while flushing initially you are still giving the fentanyl in the dead space of the line) and should chest wall rigidity occur the best treatment is administration of a fast acting muscle relaxant. Fentanyl can also be given in addition to another induction agent, usually in a dose of 1 mcg/kg, in an attempt to blunt the sympathetic response to laryngoscopy.
Morphine is used as an induction agent in some neonatal units. This practice can not be supported due to the unfavourable pharmacokinetic properties of morphine when used for this purpose (slow onset and long duration of action – the opposite of what an ideal induction agent should do), the fact that it is produces a poor level of hypnosis, the risk of hypotension due to histamine release and the fact that there are alternative more effective agents available.
Midazolam (which works by facilitating the actions of GABA) is often co-administered with another induction agent or used a premedication for it’s amnesic and anxiolytic properties during routine anaesthesia. While it can be given in a higher dose as the sole induction agent its slow onset/longer duration of action limits its use and it is also likely to cause cardiovascular instability in a critically ill child.
The Bottom Line
Due to its cardiovascular stability ketamine is the preferred induction agent for intubation of the critically ill child. It should be noted that in the shocked child, although ketamine is the safest agent to use, it is still highly likely to to cause cardiovascular collapse (due to exhaustion of endogenous catecholamines) and a reduced dose should be used. I would recommend starting with 1 mg/kg in the haemodynamically stable critically ill child and reduce to 0.5 mg/kg in the shocked child (you can always give more, but can’t take it back). Allow more time to take effect before topping up the dose in the shocked child, due to the longer time it takes the drug to reach the brain and exert its effect. To avoid errors always use the 10 mg/ml preparation or dilute the more concentrated preparation to this concentration before drawing up the dose (you will also need to use this concentration to draw up the small volumes needed for neonates accurately).
The traditional teaching that ketamine causes elevation in intracranial pressure is based on weak evidence and other agents such as propofol and thiopentone (despite their beneficial effects on reducing ICP) are more likely to cause a greater overall reduction in cerebral perfusion pressure due to the frequent hypotension experienced when using these agents, so I would still recommend the use of ketamine as the induction agent for patients with traumatic brain injury.
When intubating the patient with status epilepticus I would recommend using thiopentone as this is in line with current APLS guidelines (although propofol would be a suitable alternative). For the patient in status epilepticus who is also cardiovascularly unstable, I would probably still use ketamine to cover the intubation and then deal with the seizure afterwards.
Muscle Relaxants
The muscle relaxants can be divided into two types (depolarising and non-depolarising) based on their mechanisms of action. To understand how they work requires a basic knowledge of how transmission at the neuromuscular junction occurs. When an action potential arrives at the nerve ending it causes release of acetylcholine into the synaptic cleft (the gap between the nerve and the muscle fibre). The acetylcholine molecule transverses the synaptic cleft and binds to receptors on the post synaptic membrane causing depolarisation and muscle contraction. The acetylcholine molecule is then broken down by the enzyme acetylcholinesterase which is present in the synaptic cleft. The following video provides a great summary of this process.
Suxamethonium is the only depolarising muscle relaxant used routinely today. It’s chemical structure consists of two molecules of acetylcholine joined together and it works by mimicking the action of acetylcholine in synaptic cleft where it binds to the postsynaptic acetylcholine receptors causing depolarisation of the muscle fibre. Unlike acetylcholine it is not broken down by the acetylcholinesterase present in the synaptic cleft and it is metabolised by plasma cholinesterase in the blood. As this is a slower process depolarisation is prolonged leading to a period of muscle relaxation after the initial contraction (explaining why fasciculations precede muscle relaxation).
As their name suggests the non-depolarising muscle relaxants don’t cause depolarisation of the muscle and they work by competing competitively with acetylcholine for the postsynaptic acetylcholine receptors and by preventing acetylcholine from binding with the receptors they prevent depolarisation of the muscle fibre causing muscle relaxation. The non-depolarising muscle relaxants can be further divided into the aminosteroids (rocuronium, vecuronium and pancuronium) and the benzylisoquinoliniums (atracurium, cisatracurium and mivacurium) which helps when comparing their properties and side effects.
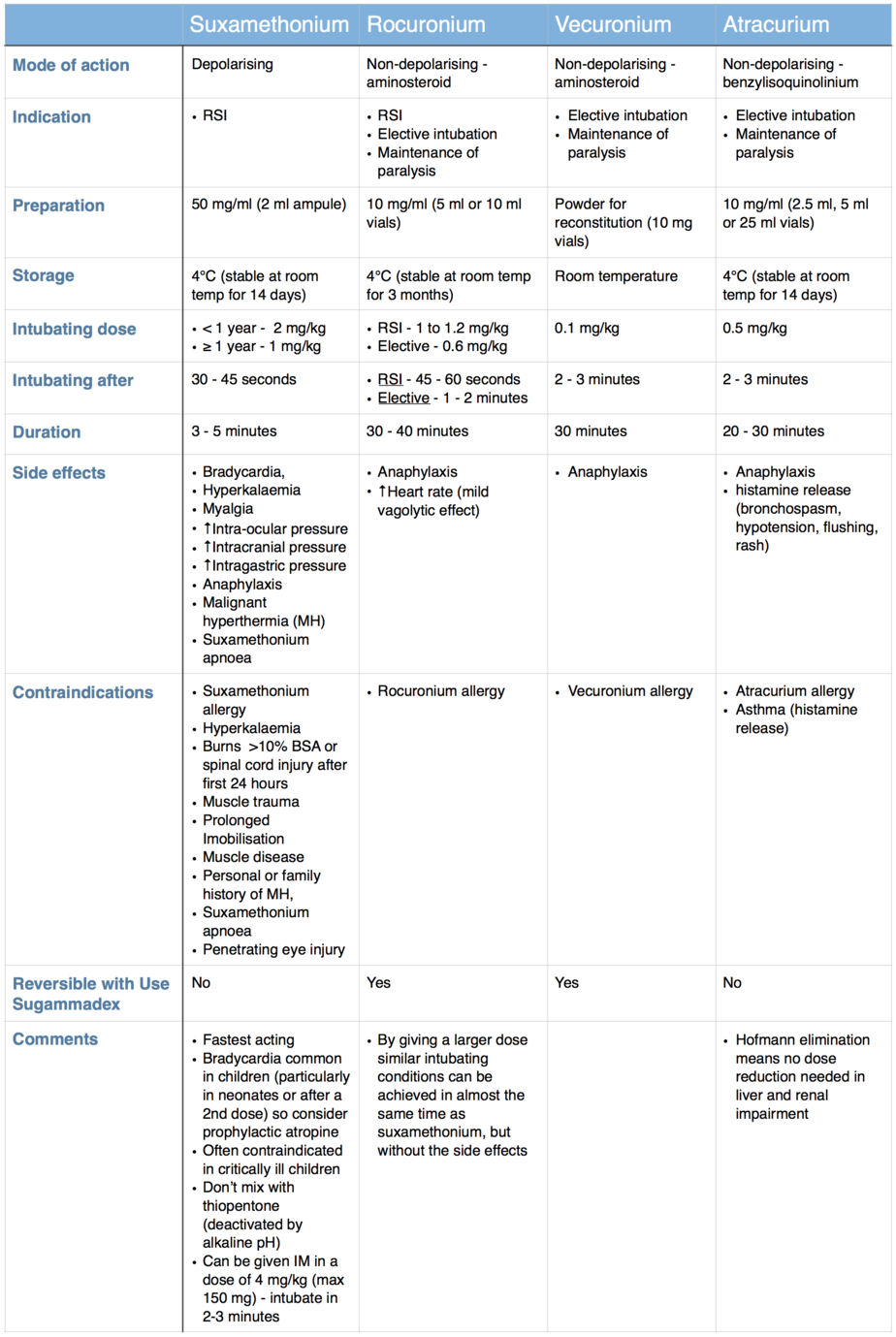
The table below compares the properties of suxamethonium and three of the most commonly used non-depolarising muscle relaxants.
Other non-depolarising muscle relaxants
Cisatracurium is one of the more potent isomers found in atracurium (atracurium is made up of 10 isomers) and as a result it is given in a lower dose of 0.15 mg/kg. Cisatracurium shares the advantageous property of Hofmann elimination with atracurium, however it has less side effects as it doesn’t cause histamine release. Cisatracurium also has a slower onset of action (3 – 4 minutes), lasts slightly longer (30 – 40 minutes) and is more expensive than atracurium.
Mivacurium is given in a dose of 0.15 – 0.2 mg/kg. It’s onset is similar to atracurium but it has a shorter duration of action lasting around 15 minutes. Histamine release is common.
Pancuronium is normally given in doses of 0.1 mg/kg and has a slow onset of action (3 – 5 minutes) and long duration of action (40 – 60 minutes). It tends to cause tachycardia and hypertension due to its sympathomimetic properties.
Muscle Relaxant Reversal
All the non-depolarising muscle relaxants mentioned above can be reversed using anticholinesterases e.g. neostigmine (given in combination with atropine or glycopyrronium to minimise muscarinic side effects). Anticholinesterases work by inhibiting the enzyme acetylcholinesterase in the neuromuscular junction, therefore increasing the amount of acetylcholine to compete with the non-depolarising muscle relaxant. Anticholinesterases won’t work, and therefore should not be given, until the effect of the muscle relaxant is starting to wear off (at least two twitches detectable on train of four). As this is normally at least 15 – 20 minutes following administration of most non-depolarising muscle relaxants this limits their usefulness in the setting of failed intubation and their main use is in the operating theatre to reverse residual neuromuscular block at the end of a case.
Thankfully there is however a method of reversing certain non-depolarising muscle relaxants immediately after they have been given. Sugammadex is a selective relaxant binding agent that encapsulates the aminosteroid group of muscle relaxant preventing their action (these complexes are later excreted by the kidneys). If a dose of 16 mg/kg dose is given immediately after the administration of an aminosteroid neuromuscular blocking agent (rocuronium, vecuronium or pancuronium), recovery can be expected within 1 – 3 minutes. It must be remembered that Sugammadex will have no effect on the benzylisoquinoliniums e.g. atracurium, cisatracurium and mivacurium.
The Bottom Line
While the above classification of the muscle relaxants into depolarising/non-depolarising and aminosteroids/benzylisoquinoliniums is important knowledge that should be known by all who use these drugs, what really determines their usefulness when it comes to intubating the critically ill child is their onset of action. Even though a classical rapid sequence induction is rarely performed in a critically ill child, as it is common practice to provide gentle face mask ventilation during the apnoea period prior to intubation (while waiting for the muscle relaxant to take effect), the speed of onset of the muscle relaxant is no less important. By choosing a muscle relaxant with a shorter onset of action you can minimise the duration of face mask ventilation and the complications that go with it (failed or inadequate face mask ventilation leading to hypoxia, gastric distention impairing ventilation and increasing risk of aspiration, time wasted that could be spend doing another task). Only suxamethonium and an RSI dose of rocuronium provide good intubating conditions in under one minute, while all the other muscle relaxants mentioned above take at least two minutes to provide similar intubating conditions. Therefore if you don’t want to have to provide face mask ventilation for longer than necessary in a critically ill child your choice of muscle relaxant is limited to either suxamethonium or rocuronium.
When you consider that rocuronium produces similar intubating conditions only 15 seconds slower than suxamethonium, but has significantly less side effects/contraindications and can be reversed with sugammadex means that I would recommend use of rocuronium as the muscle relaxant of choice for intubating the critically ill child. I would also recommend using a dose of 1 mg/kg for ease of calculation (0.1 ml/kg of a 10 mg/ml solution).
Suxamethonium may be preferred in certain circumstance such as intubation of neonates for surfactant therapy using the INSURE method (INtubation-SURfactant-Extubation) due to it’s duration of action. Similarly if sugammadex is not available and the patient is anticipated to have a difficult airway or in the situation where senior support for the intubator is not immediately available the advantages of the shorter duration of action of suxamethonium may outweigh the side effect.
Rescue Medication
The stimulating effect from the physical process of laryngoscopy or the presence of hypoxia occurring during the intubation attempt can both induce a vagally mediated bradycardia. Also considering many of the drugs administered to facilitate intubation have bradycardia as a side effect means you must be prepared to deal with this predictable complication.
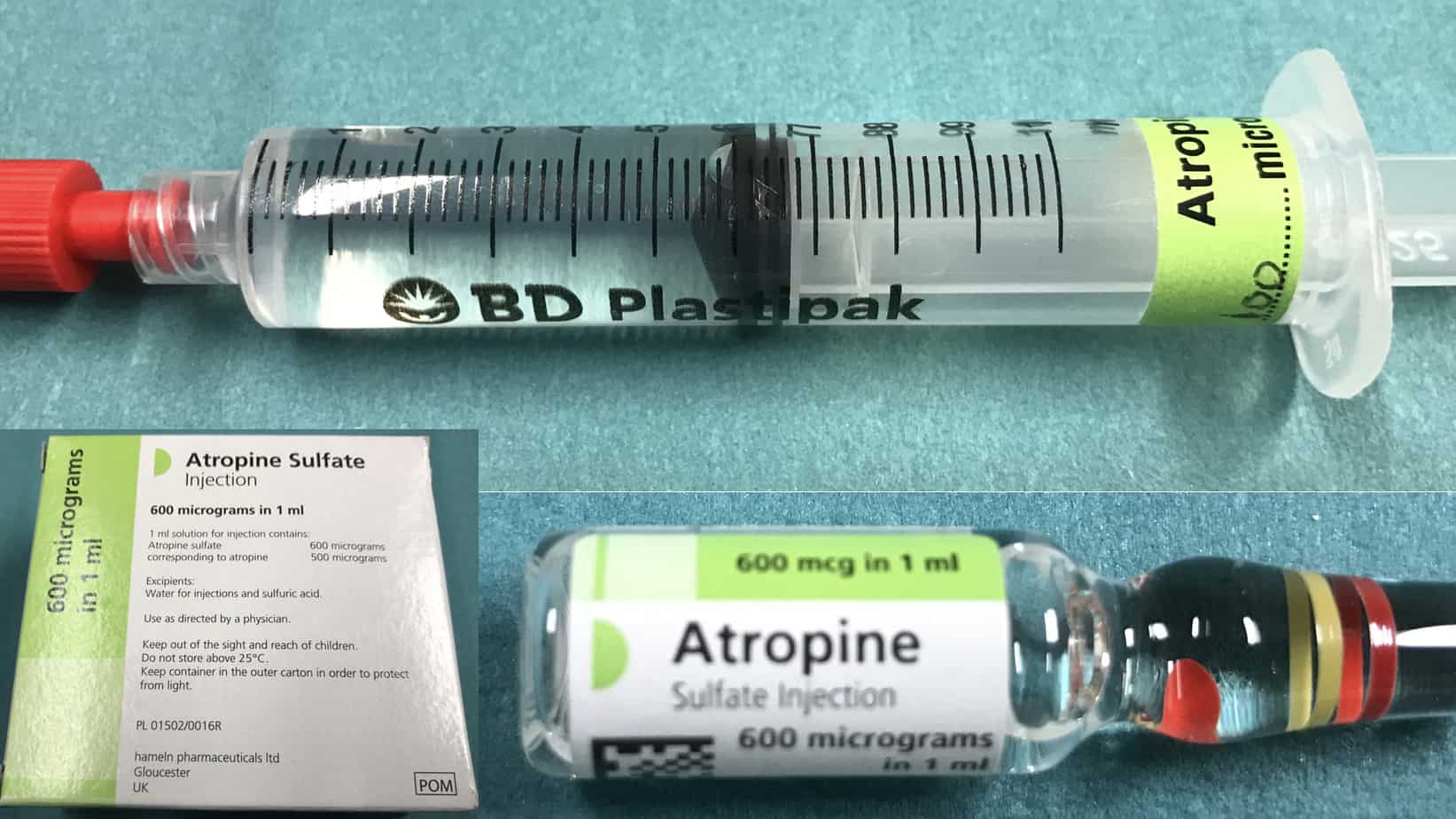
Atropine should be prepared in advance for every intubation (although it only takes around 30 – 60 seconds to draw up the dose in an emergency, this is a very long time when watching a bradycardic child). For treatment of a vagally mediated bradycardia atropine is given in a dose of 20 mcg/kg (max 600 mcg). In high risk patients atropine can be given electively on induction to try and prevent bradycardia from occurring. For prophylaxis the same dose is used as for treatment of bradycardia except that in infants ≥ 1 month a minimum dose of 100 mcg is used as smaller doses can be associated with paradoxical bradycardia. Atropine comes in 1 ml ampules containing 600 mcg (600 mcg/ml) or in prefilled syringes of various concentrations (100 mcg/ml, 200 mcg/ml, 300 mcg/ml). For ease of calculation and to help avoid errors I would recommend than you either use the 100 mcg/ml prefilled syringe or dilute all the 1 ml ampule of atropine (600 mcg/ml) to 6 ml with 0.9% saline to make a solution of 100 mcg/ml (working in multiples of 10 simplifies the required calculations).
As already mentioned all the induction agents have the potential to cause cardiovascular collapse in the critically ill child. Even with the best efforts to prevent this from occurring by for example reducing the dose of the induction agent and by attempting to optimise the haemodynamics prior to induction of anaesthesia, cardiovascular instability can’t always be prevented and you need to have a plan to deal with this when it occurs. It is normally managed by a combination of fluid boluses and ‘Push Dose Pressors’.
Fluid boluses need to be given rapidly to be effective and in children the required aliquot of fluid (normally 10 – 20 ml/kg) should be drawn up into a syringe and pushed in by hand. As drawing up the fluid takes time, if you feel your child is likely to need a fluid bolus to treat hypotension on induction it makes sense to have the required volume already drawn up prior to starting. For unanticipated hypotension where this hasn’t been done a bag of appropriate fluid e.g. 0.9% saline, a bag spike (allows rapid filling of the syringe) and 50 ml syringes should be immediately available.
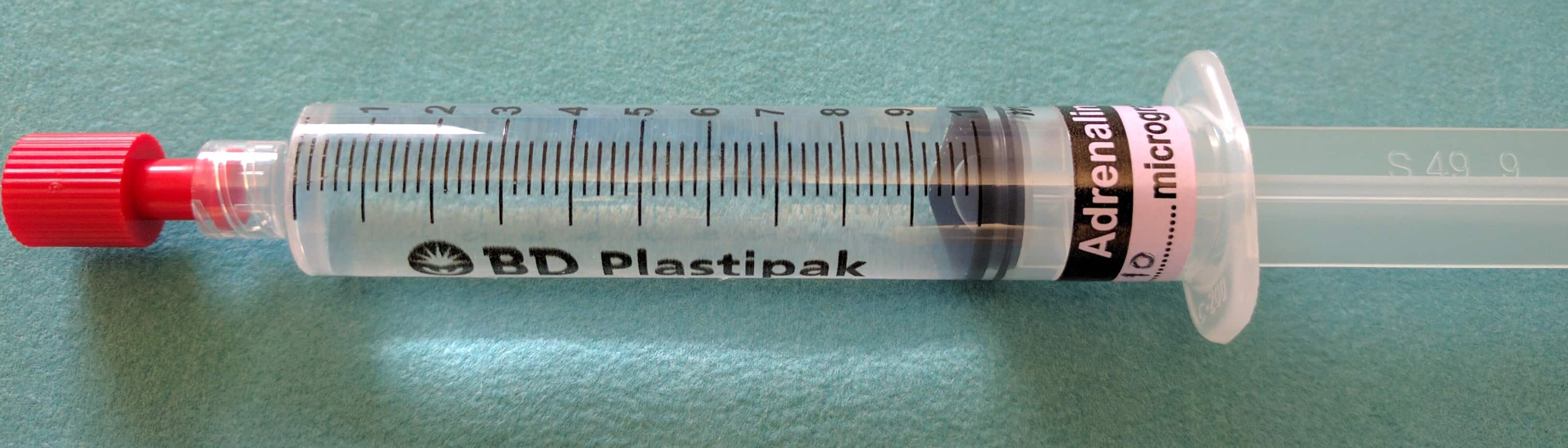
Normally vasoactive drugs are given in concentrated infusions via a central line. In contrast ‘Push Dose Pressors’ are titrated by administering small aliquots from a dilute vasoactive drug syringe by bolus injection, normally via a peripheral line. Due to the speed at which they can be initiated ‘Push Dose Pressors’ are used primarily in the crashing peri-arrest patient to obtain stability, where starting a standard infusion of the drug would take too long (they buy time while a standard vasoactive drug infusion is prepared and started) or where the duration of hypotension is expected to be short lived, making a standard infusion unnecessary e.g. hypotension following induction of anaesthesia. There are a number of drugs that are commonly used as ‘Push Dose Pressors’ but as adrenaline is one of the most effective, best suited to the crashing child and is easily available on every resuscitation trolley in the hospital, it is the one I would recommend using.
Instructions for Preparing Push Dose Adrenaline

Post Intubation Drugs
Following intubation it should be remembered that the effects of the muscle relaxant will persist for longer than induction agent (unless suxamethonium is used) and maintenance sedation should be started as soon as possible (to prevent the situation when the child is consciousness but paralysed). Should the planned duration of intubation be expected to be short e.g. intubated only to facilitate a procedure or for status epilepticus with a plan to wake and extubate, this is best done with further boluses of the induction agent given as required.
However In most cases when the critically ill child is intubated a trip to intensive care is required, and as the sedation will be required for a longer period, a continuous infusion is more suitable for this purpose. Sedation preferences varies widely from area to area and the advice below is just one approach.
The most commonly used agent for sedation in critically ill children is morphine. See below for details on how make up a continuously infusion.
Morphine Infusion
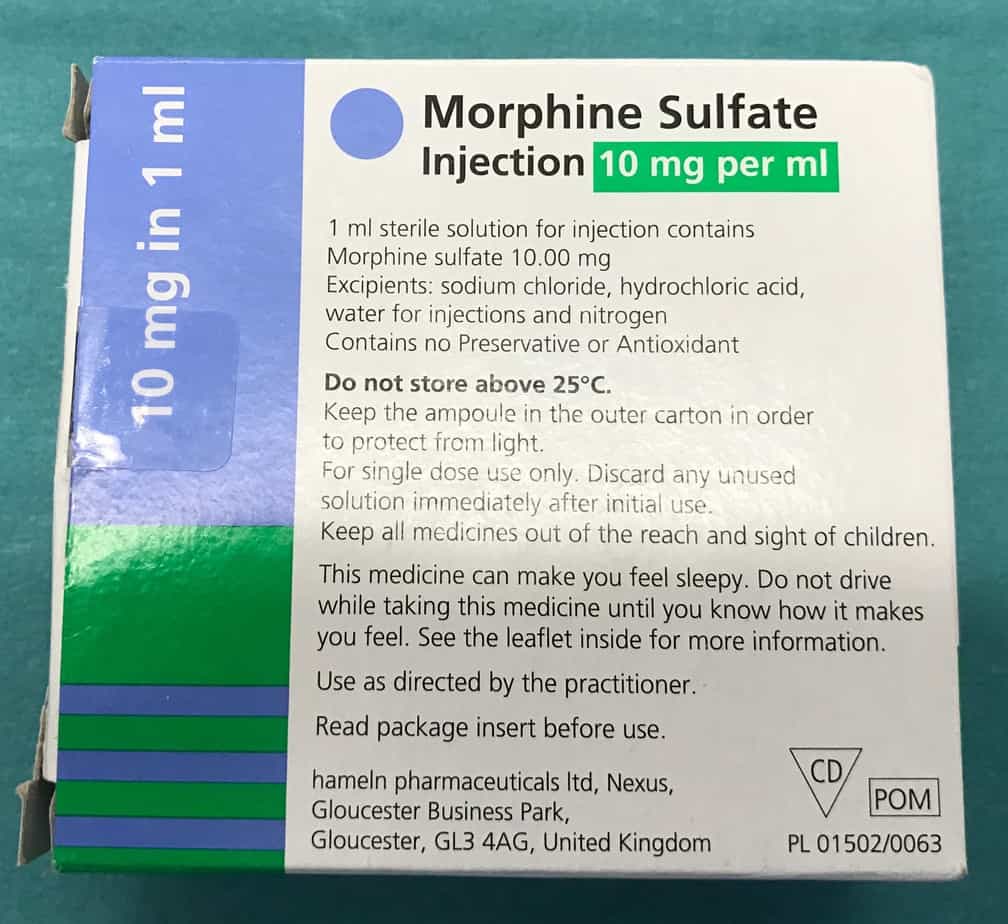
- Reconstitution: child’s weight in kg mg of morphine in 50 ml of 0.9% saline e.g. 18 mg in 50 ml of 0.9% saline for an 18 kg child
- Starting dose: 1 ml/hr = 20 mcg/kg/hr and titrate to effect
- Range: 0.5 – 3 ml/hr (10 – 60 mcg/kg/hr) – lower upper limit in neonates
- Loading dose: Administer 20 – 100 mcg/kg (1 – 5 ml of the reconstituted infusion) as a loading dose depending on haemodynamics and requirement – suggest giving loading dose in 1 – 2ml (20 – 40 mcg/kg) aliquots.
In infants less than 3 months morphine alone should be adequate for sedation and addition of midazolam in most cases is not only unnecessary, but is frequently associated with the side effects hypotension, over sedation and withdrawal. In infants over 3 months of age midazolam is often required in addition to morphine to achieve satisfactory levels of sedation. See below for details on how to make up a midazolam infusion.
Midazolam Infusion

- Reconstitution: 5 x child’s weight in kg mg of midazolam in 50 ml of 0.9% saline e.g. 90 mg in 50 ml of 0.9% saline for an 18 kg child
- Starting dose: 1 ml/hr = 0.1 mg/kg/hr and titrate to effect
- Range: 0.6 – 2.4 ml/hr (0.06 – 0.24 mg/kg/hr)
- Loading dose: Administer 50 – 100 mcg/kg (0.5 – 1 ml of the reconstituted infusion) as a loading dose depending on haemodynamics and requirement (suggest giving loading dose in 0.5 ml aliquots).
Other than local preference the main reason to deviate away from the above recommendations occurs in asthma where morphine administration may worsen bronchospasm due to histamine release. In this setting the morphine can be replaced with either a fentanyl or ketamine infusion (ketamine is probably a better choice due to its bronchodilatory properties). While commonly used for sedation in adult intensive care it is important to remember that propofol infusions are contraindicated for sedation in children due to this risk of propofol infusion syndrome.
If required, paralysis can be continued with boluses of muscle relaxant given as required or by starting a continuous infusion. The biggest determiner of which non-depolarising muscle relaxant to use tends to be local preference. However if the patient has renal or hepatic failure using atracurium makes sense due to Hofmann elimination and likewise atracurium should be avoided in asthmatics due to risk of histamine release worsening bronchospasm. As I use rocuronium for intubation, unless there is a good reason to use another agent, I also use rocuronium for maintenance of paralysis when required.
Rocuronium Infusion
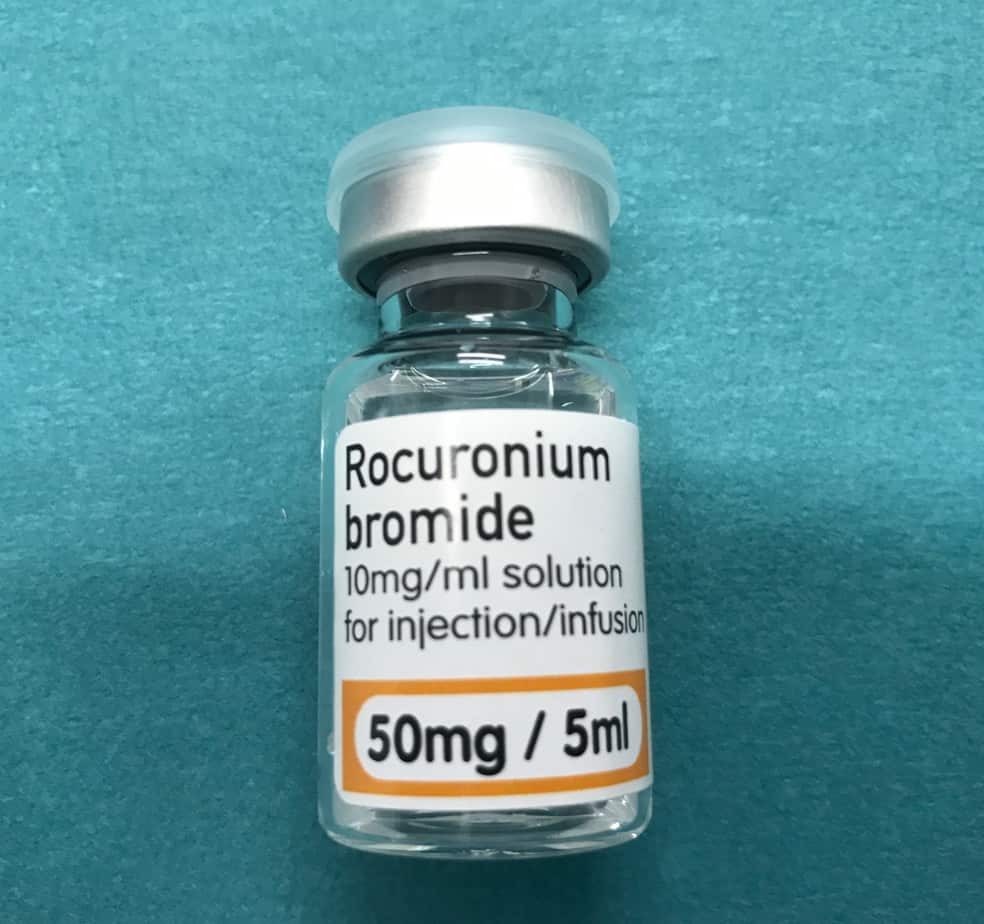
- Reconstitution: 500 mg of rocuronium in 50 ml neat
- Starting dose: 0.05 x child’s weight in kg ml/hr = 0.5 mg/kg/hr and titrate to effect e.g. 0.9 ml/hr for an 18 kg child
- Range: 0.3 – 1 mg/kg/hr
- Bolus/loading dose: Administer 0.6 mg/kg as a loading dose
One advantage of bolus administration is it allows assessment of neurology and depth of sedation between doses (particularly important if at risk of seizures), while the continuous infusion provides more stability. Most children will be kept paralysed if requiring external transfer to PICU as this helps facilitate a safe transfer, however following arrival in PICU muscle relaxants will be used for the minimum amount of time necessary due to concerns with side effects and are often stopped on admission.
![]()
